MRLine
MR CONDITIONAL MEDICAL DEVICES
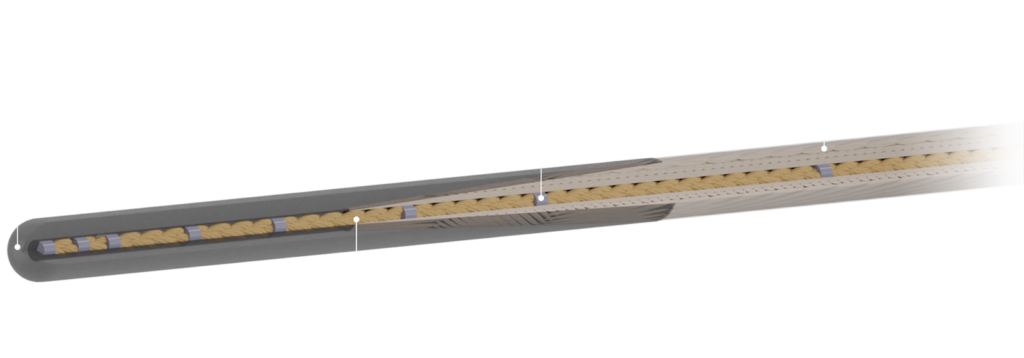
MRline – A new era in guide wire technology.
Our CE marked guide wire enables interventions in MRI and
meets all MRI safety standards. This opens new opportunities in this field.
ADVANTAGES
- enables interventions in MRI
- meets all MRI safety standards
- navigation through discrete markers
- available in 0.035”
- non-breakable corefibres
- good steerability
- crocodile® markers
- non-conductive
- we produce wires according to your specifications
- FDA approval in progress
SAFETY INFORMATION
A patient with this device can be safely scanned in an MR system meeting the following conditions:
- static magnetic field of 1,5 Tesla and 3 Tesla, with
- maximum spatial field gradient of 12,800 G/cm (128 T/m)
- maximum Force product of 231,000 G2/cm (231 T2/m)
- theoretically estimated maximum whole body average (WBA) specific absorption rate (SAR) of
2 W/kg (Normal Operating Mode)
4 W/kg (First Level Controlled Operating Mode)
Publications
Innovative EPflex medical devices for endomyocardial biopsy open up new potential for interventional cardiology in MRI
Magnet resonance imaging (MRI) has decisive advantages compared to fluoroscopic guidance: not only is it possible to characterize soft tissue in much greater detail and without the use of harmful contrast agents, but it also operates without the damaging radiation of X-rays. In cardiology in particular, the high soft tissue contrast has the advantage that pathological or inflamed tissue in the heart can be identified fast and precisely. For further examination of the tissue, it is still necessary to take e.g. a tissue sample from the heart wall (endomyocardial biopsy), but this is currently only possible subsequently under X-ray imaging, as the minimally invasive, highly specialized medical devices for interventional cardiology in MRI (iCMR) are still non-existing. EPflex has risen to this challenge.
The choice of materials for medical devices for use under MR imaging is severely limited: magnetic materials must be completely avoided and metallic but non-magnetic materials must be carefully examined for heating and image artefacts. The innovative challenge of the EPflex development of an MRI-compatible biopsy forceps was therefore to achieve the same mechanical performance of conventional, all-metal bioptomes but with a virtually metal-free design based on fiber composites and high-performance polymers.
In 2021, EPflex and its cooperation partner ICCAS successfully completed the BMBF-funded research project to develop an MRI-compatible bioptomes (EPflex) and the associated clinical workflow and imaging (ICCAS) with a proof-of-concept study.
In the years that followed, EPflex further developed the design of the bioptomes so effectively that the next generations of bioptomes could now be analyzed in a combined in-vivo and ex-vivo study for performance, safety, and clinical viability for endomyocardial biopsy in MRI.
At the University Medical Center Göttingen, under the direction of Dr. Christina Unterberg-Buchwald and in cooperation with Angelika Svetlove from the Max Planck Institute for Multidisciplinary Sciences, Göttingen, various designs of the EPflex bioptomes were initially investigated ex-vivo by taking samples from the pig heart wall using two forceps blade geometries in a controlled experimental setup. Using phase-contrast synchrotron radiation computed microtomography (SRµCT) and conventional two-dimensional histology, the samples were evaluated for volume, inner and outer integrities, compression and histological diagnostic value. These results showed that the EPflex bioptomes achieve similar results to conventional bioptomes.
Furthermore, the bioptome designs were tested in-vivo on mini pigs as part of a DZHK project. The clinical feasibility of heart biopsy with the new EPflex bioptomes was measured by procedural and cutting success as well as histological diagnostic value of the samples taken. All three animal studies were carried out without complications and usable tissue samples were successfully extracted from the heart wall.
The results show that the new MR-compatible bioptomes are promising and have the potential to improve the diagnostic accuracy of endomyocardial biopsy. However, further studies are required to verify their clinical suitability.
Following the world’s first MRI-conditional guidewire (CE-marked since 2012), EPflex now has the second revolutionary iCMR innovation in the starting blocks, with which we are pushing the boundaries of minimally invasive surgery a little further.
Author: Dr. Senta Schauer
The results of the study were recently published in the journal European Radiology Experimental:
Svetlove, A. et al. Evaluation of MR-safe bioptomes for MR-guided endomyocardial biopsy in minipigs: a potential radiation-free clinical approach. Eur Radiol Exp 7, 76 (2023).
https://doi.org/10.1186/s41747-023-00391-4
We thank all those involved in the publication, especially Angelika Svetlove and Christina Unterberg-Buchwald, for their successful collaboration. We would also like to thank our project partners at ICCAS and the Federal Ministry of Education and Research BMBF for their support (funding reference 13GW0242A).
Your Contact Person
Dr. Senta Schauer
Director of R&D
T +49 7123 9784-0
